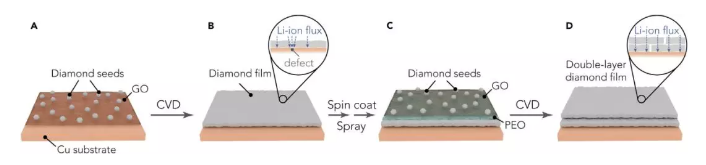
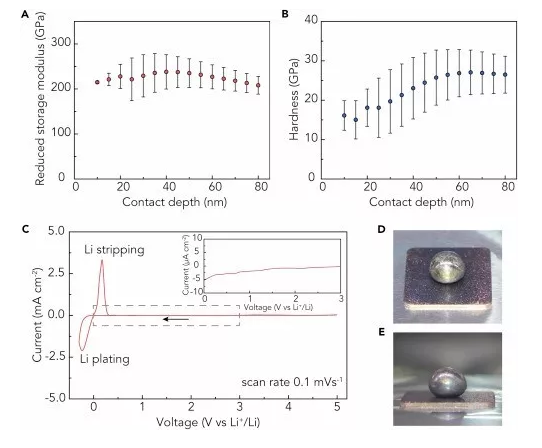
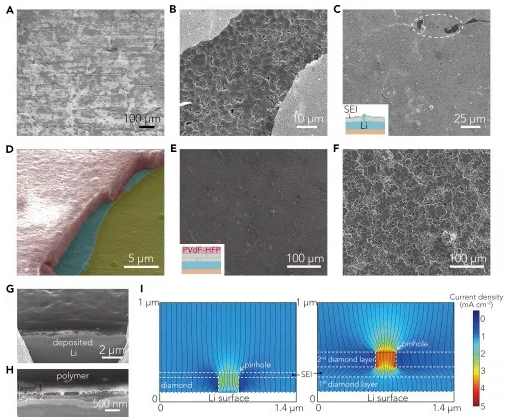
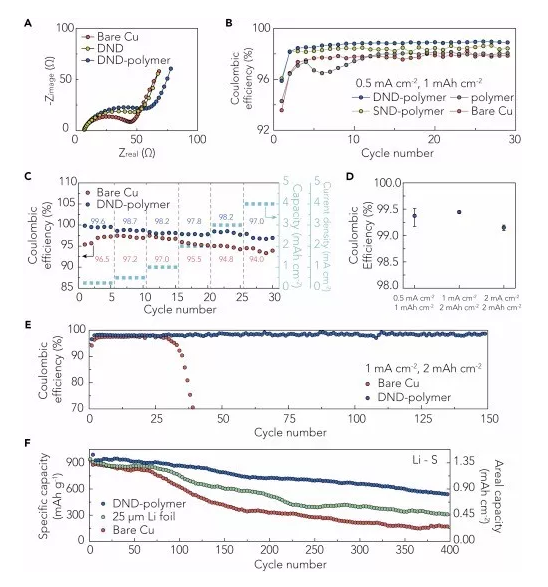
Recently, Professor Cui Wei and Prof. Zhu Yuwen (communication author) of Stanford University in the United States reported a super-strong double-layer nano-diamond interface layer, which can greatly improve the stability of Li metal negative electrode. This work was the first use of diamond in the design of the interface layer because of its good mechanical strength and electrochemical inertness, which can be used for Li metal coatings. The Li metal interface layer designed in this work has the highest elastic modulus (>200GPa) in all currently reported Li metal coatings, which can effectively inhibit the growth of Li dendrites and the generation of side reaction on the electrode surface. The volume change of Li metal during the cycle. In order to solve this problem, the artificial SEI layer will generate small holes in the surface of the Li battery to promote Li+ flux and dendrite growth. This work proposes a new two-layer structure design, which greatly improves the defect tolerance. It is able to achieve uniform Li+ flux and mechanical properties, which was confirmed in simulation and experiments. Therefore, this nano-diamond interfacial layer exhibits excellent electrochemical performance: Coulomb efficiency exceeds 99.4% at 1 mA·cm-2; in typical Li-S batteries, it has excellent cycle stability over 400 laps. It is also possible to maintain a high specific capacity, and the average coulombic efficiency of the negative electrode exceeds 99%. The related results were published on Joule under the heading "An Ultrastrong Double-Layer Nanodiamond Interface for Stable Lithium Metal Anodes". ã€Research Background】 Li metal has a high theoretical specific capacity (3,860 mAh·g-1) and a very low electrochemical potential (-3.040 V, compared to a standard hydrogen electrode), making it an ideal anode material for next-generation lithium-ion batteries. However, Li metal has high reactivity, and almost any available electrolyte can form a solid electrolyte membrane (SEI) on the surface of Li, and this SEI layer has poor mechanical properties and cannot withstand mechanical deformation of the electrode during the cycle. It is prone to cracks, causing the growth of Li dendrites, causing internal short circuits and causing serious safety problems. In addition, repeated formation and destruction of the SEI results in continuous loss of Li metal and electrolyte, resulting in low coulombic efficiency (CE) and rapid capacity decay. Therefore, it is urgent to construct a stable SEI layer to suppress the growth of Li dendrites and the generation of side reaction on the electrode surface. In the design of the interface layer and the selection of materials, the following requirements should be met: (1) inertness to Li metal, which excludes most polymer and inorganic coatings; (2) high elastic modulus Amount and compact structure, which is essential for inhibiting the growth of Li dendrites; (3) requires a certain degree of flexibility to accommodate the volume change of the electrode during the cycle; (4) can maintain a uniform flow of lithium ions, will not How much local Li+ flux occurs; (5) requires low conductivity and weak interaction with the substrate, so that Li deposition can be performed under the film layer. [Research Highlights] This work is in the design of the Li metal interface layer: in the material selection, the diamond material was selected for the first time because of its high modulus of elasticity, chemical inertness and electrical insulation properties, which provides a super strong for Li metal. The interface layer; in the structural design, the double-layer structure design achieves uniform Li+ flux and mechanical properties, and strictly meets all the above requirements. In the material selection and structural design, it provides a new idea for the design of Li metal interface layer. [Graphic interpretation] Figure 1 Design principle and synthesis steps of nano-diamond interface layer (A) the Cu substrate is first pretreated with a GO release layer and sprayed with colloidal diamond seed particles; (B) growing a single-layer nano-diamond film (SND) by an MPCVD method; (C) coating a layer of PEO (polyethylene oxide) protective layer on the SND, and then spraying the colloidal diamond seed particles on the protective layer, the process is the same as (A); (D) A two-layer nano-diamond (DND) film obtained by MPCVD treatment. [Points] Ø Selecting the Cu substrate and the GO release layer can produce a nano-diamond film with low defect density; Ø It is difficult to eliminate mechanical damage to the film during CVD, battery assembly or recycling. These defects can locally increase the flow of lithium ions and easily cause the growth of Li dendrites; Ø In the subsequent MPCVD process, on the one hand, the PEO layer can protect the first layer of nano-diamond film from damage, on the other hand also acts as a template layer, which will be melted and etched away by the plasma, in two layers of nano-diamond film. Form a tiny gap between them; Ø DND is designed so that the defects of the underlying film can be shielded by the top layer of the nano-diamond film layer, which greatly improves the defect tolerance of the nano-diamond interface layer and promotes the uniformity of the mechanical properties of the lithium ion flow and the nano-diamond interface layer. Figure 2 is a schematic representation of the characterization of the nano-diamond interface layer (A and B) low (A) and high (B) SEM images of DND; (C) TEM image of the nano-diamond film, clear nanopores can be observed in a thinner region; (D) a photograph of a DND film grown on a copper substrate; (E) SEM false color map of the cross section of the nano-diamond interface layer, which has a distinct two-layer structure; (F) DND high-magnification SEM false color map, which proves that the defects of the top nano-diamond film layer (blue region) can effectively protect the underlying nano-diamond film layer (yellow region); (G) Raman spectra of Cu substrate, SND film and DND film after pretreatment with GO layer and without spraying seed particles. [Points] Ø Low-power SEM images show that continuous nano-diamond films can grow to the order of a few hundred microns, which is much larger (usually less than 10 μm) than the previously reported two-dimensional material-based Li metal interface; The SEM image shows that the film is a compact and flat structure composed of diamond nanocrystals; Ø TEM image confirms the nanoporous nature of polycrystalline nanodiamond film, which is essential for transporting Li+ at the interface; (D) shows that the nanodiamond film is homogeneous in a large region due to light Judging by the luminescence exhibited by the scattering of nanodiamond crystal grains; Ø From the SEM image of the cross section, the thickness of each layer of nano-diamond film can be measured to be about 150 nm. The interlayer gap is left after the removal of the PEO template layer, and has a clear double-layer structure; Ø Comparing the Raman spectra of the three, it can be found that the high content of sp3 carbon is essential to ensure the mechanical and electrochemical properties of the interface layer, and the nano-diamond interface layer designed by this work has a unique sp3 peak, indicating The CVD method can obtain a high quality nano diamond film. Figure 3. Advantages of nano-diamond interface layer on Li metal stability (A and B) reduced storage modulus (A) and hardness (B) of DND, obtained by nanoindentation measurement; (C) CV curve of nanodiamond, scanning speed is 0.1 mV·s-1; (D and E) illustrate photo top view (D) and side view (E) of wet Li on the nanodiamond film. [Points] Ø The weak interaction between the nano-diamond film and the substrate and its nano-porous properties facilitate the growth of Li dendrites under the protective film; the extremely low conductivity of this material prevents Li from directly nucleating on the nano-diamond film; The film has good flexibility and can be well adapted to the volume change of Li metal during the cycle; Ø Due to the high carbon content of the nano-diamond interfacial layer sp3, it has high mechanical strength, can effectively inhibit the growth of Li dendrites, and can hinder the parasitic reaction during the cycle, showing high electrochemical performance; The wettability of the diamond interface layer is very poor, which is favorable for uniform deposition of Li and inhibits dendrite growth. Figure 4. Deposition of Li on the interface layer of nanodiamond (A and B) SEM images of Li deposited on the SND electrode (A) and the electrode (B) after removing part of the SND at a current density of 0.5 mA·cm-2 for 2 h; (C) a scaled SEM image of the SND electrode after Li deposition, wherein the circled area represents the Li protrusion due to the local defect, and the inset is a schematic view of the deposition morphology of the defect area; (D) SEM image of a cross section of the DND-polymer structure, wherein the pink region represents the PVDF-HFP layer, and the green and yellow regions correspond to the top and bottom of the nanodiamond layer, respectively; (E and F) SEM images of DND-polymer electrode (E) and Cu electrode (F), cycled for 20 cycles at a current density of 1 mA·cm-2 and a capacity of 1 mAh·cm-2; (G and H) Cross-section FIB-SEM image of Li deposition on DND-polymer electrode, current density 1 mA·cm-2, capacity 1 mAh·cm-2, (G) FIB-SEM image of low magnification The morphology of the Li deposition and the (H) high magnification FIB-SEM image showing the complete structure of the DND-polymer coating; (I) Simulation of lithium ion current through SND (left) and DND (right) in a 200 nm pinhole with a nominal current density of 1 mA·cm-2, streamlined Li+ flux, perpendicular to Li metal surface . [Points] Ø After deposition of Li on SND electrode for 2h, it is found that there is not much Li overgrowth in a large area, and the defect density is not increased, which confirms the flexibility of the nano-diamond film and can adapt to the volume change of Li metal; After that, a compact Li deposition morphology can be seen, further confirming that the nano-diamond interfacial layer can inhibit the growth of Li dendrites and the side reaction between Li metal and electrolyte; Ø However, there are local defects in SND. It can be seen that a small amount of Li protrudes at the defect, so a double-layer design is carried out to greatly improve the defect tolerance of the nano-diamond interface layer, so it is looped 20 times compared with the Cu electrode. The rear electrode can still maintain a smooth surface, that is, the growth of Li dendrites does not occur; Ø COMSOL simulation further confirms that the double-layer design can improve the uniformity of Li+ flux. The presence of small holes of about 200 nm in SND will increase the local Li+ flux and promote the formation of Li dendrites, while the second in DND. The layered nano-diamond film can maintain a constant Li+ flux and achieve uniformity of Li deposition. Figure 5. Characterization of electrochemical properties of nano-diamond interfacial layer (A) electrochemical impedance spectroscopy of Cu, DND and DND-polymer electrodes; (B) Coulombic efficiency of different negative electrodes, current density of 0.5 mA·cm-2 and capacity of 1 mAh·cm-2; (C) Coulombic efficiency of Cu and DND-polymer electrodes under different current density and capacity cycling conditions; (D) Average coulombic efficiency after 10 cycles of DND-polymer electrode; (E) Coulombic efficiency after long-term cycling of Cu and DND-polymer electrodes, current density of 1 mA·cm-2 and capacity of 2 mAh·cm-2; (F) Cyclic performance of a typical Li-S battery at 0.5 C. [Points] Ø Electrochemical impedance spectroscopy is used to evaluate the charge transfer resistance of the nano-diamond electrode. The impedance of the Cu electrode is about 39 Ω, while the impedance of the DND-modified electrode is about 49 Ω. This is due to its nano-porous nature and the ability of Li+ to be easily worn. Through the nano-diamond interface layer; and the additional polymer coating does not deteriorate the impedance of the electrode (~50 Ω), maintaining the stability of Li metal; Ø Figure B shows that the coulombic efficiency of the Cu electrode is relatively reduced (~97.5%), which is mainly caused by the uneven formation of Li deposition and the repeated formation of the brittle SEI layer; the Coulomb efficiency of the polymer-coated Cu electrode is in the subsequent cycle. The increase is small (~0.2%), mainly due to the trapping of the electrolyte by the polymer to reduce the occurrence of parasitic reactions; however, the coulombic efficiency of the SND-polymer modified Cu electrode reaches 98.5%, indicating that the nanodiamond interfacial layer promotes The uniformity of Li deposition reduces the direct contact between Li metal and electrolyte; the average coulombic efficiency of DND-polymer modified Cu electrode is 99%, indicating the advantages of double layer design of interface layer; Ø The coulombic efficiency measured at different current densities indicates that the coulombic efficiency of the DND-polymer modified Cu electrode is about 3% higher than that of the unmodified Cu electrode. The subsequent test results also indicate DND-polymer modification. The Cu electrode maintains good cycle stability and the Coulomb efficiency decay is small; Ø Li-S battery cycle test performance shows that when the Cu electrode is used as the negative electrode, it starts to decay rapidly after 75 laps; the 25 μm lithium foil battery also shows rapid decay after 90 laps; while the DND-polymer battery shows very Good cycle stability, still has a high capacity retention rate at 400 laps, which indicates that the nano-diamond interfacial layer can effectively inhibit the shuttle effect in Li-S cells and improve electrochemical performance. [Summary and Outlook] Li metal anodes are used as cathodes for next-generation lithium batteries, and interfacial stability between Li-electrolytes is critical. This work rationally designed a high-quality nanoporous diamond interfacial layer with weak interaction between the current collector and low conductivity and high electrochemical stability, which enables Li to deposit under the interface layer. The parasitic reaction between Li metal and electrolyte is reduced; the high elastic modulus (>200 GPa) of the interfacial layer can effectively inhibit the growth of dendrites and achieve dense and uniform Li deposition after long-term circulation; The new two-layer design greatly increases the defect tolerance of nanodiamond films. These advantages of the nano-diamond interface layer make it exhibit excellent electrochemical performance in half-cell and Li-S full cells. Finally, this work provides new ideas for material selection and structural design of the Li metal interface layer. [Document information] An Ultrastrong Double-Layer Nanodiamond Interface for Stable Lithium Metal Anodes, (Joule, 2018, DOI: https://doi.org/10.1016/j.joule.2018.05.007)
Shower Light Fixture,Modern Bathroom Lighting,Led Shower Light,Bathroom Ceiling Light NINGBO EASTKEY ILLUMINATE APPLIANCE CO.,LTD , https://www.dkledmirrorlight.com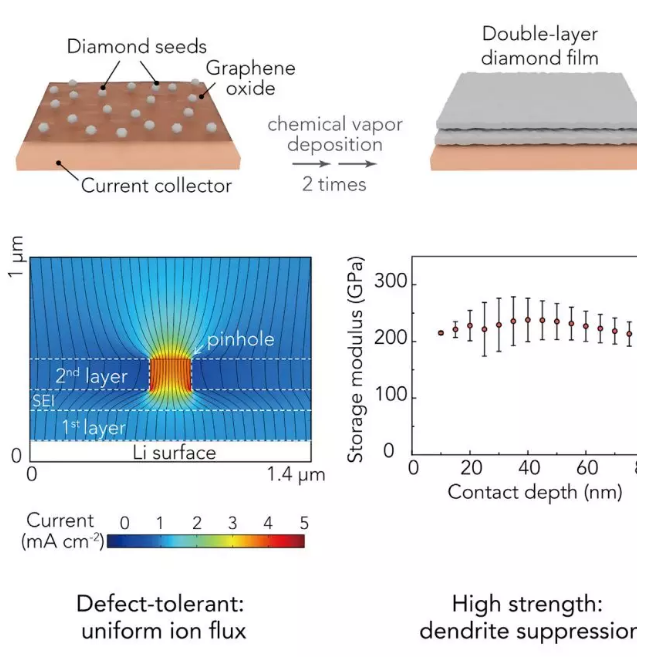

Abstract Recently, Professor Cui Wei and Prof. Zhu Yuwen (communication author) of Stanford University in the United States reported a super-strong double-layer nano-diamond interface layer, which can greatly improve the stability of Li metal negative electrode. This work in the design of the interface layer, the first use of diamond material, this ...