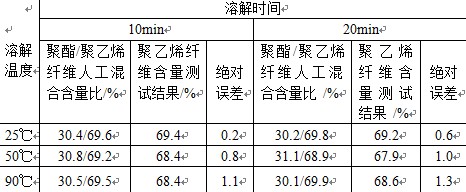
In the routine fiber content inspection work, it is found that some textiles currently have polyester fiber and polyethylene fiber blended, but GB/T2910-2009 "Textile Quantitative Chemical Analysis" and other commonly used textile testing standards do not provide corresponding Quantitative method. To this end, this paper combines the experience of practical work to explore the quantitative method of this blended product. 1 test principle and steps 1.1 sample A known ratio of polyester fiber/polyethylene fiber mixture artificially mixed. 1.2 reagent Quantitative Analysis Reagents: In order to maximize the usability and operability of the method, reagents for quantification are sought to be selected from the most commonly used reagents in the laboratory. It can be seen from the textile industry standard FZ/T01057.4-2007 "Textile fiber identification test method Part 4: Dissolution method" that the solubility of polyester fiber and polyethylene fiber in 98% concentrated sulfuric acid is different. Polyester fiber is soluble in concentrated sulfuric acid but polyethylene fiber is insoluble, and concentrated sulfuric acid is a commonly used reagent, which is easy to obtain. Therefore, we choose concentrated sulfuric acid with a mass fraction of 98% as a reagent for quantitative analysis. Residue washing neutralization reagent: Dilute ammonia solution of 4.2 in GB/T2910.11—2009, that is, 80mL concentrated ammonia water (Ï=0.880g/mL) was diluted with water to 1L. A sulfuric acid solution having a mass fraction of 75%: 700 mL of concentrated sulfuric acid (Ï = 1.84 g/mL) was added to 350 mL of water, and after the solution was cooled to room temperature, water was added to 1 L. 1.3 Test principle The polyester fiber is dissolved and removed from the mixture of known dry mass by concentrated sulfuric acid with a mass fraction of 98%. The residue is collected, washed, dried and weighed to calculate the mass percentage of the polyethylene fiber. The content of polyester fiber. 1.4 Test conditions Dissolve the mixture in 2.1 at a dissolution temperature of 25 ° C, 50 ° C, 90 ° C for 10 min, 20 min, and make 10 parallel samples for each test condition. The average of 10 results is used as the final result. A comparison of the final results obtained from the test conditions to find the best test conditions. 1.5 test steps Prepare the sample according to the procedure specified in GB/T 2910.1-2009 "Quantitative Chemical Analysis of Textiles Part 1: General Rules of Testing", then follow the steps below: Place the prepared sample into an Erlenmeyer flask, add 100 ml of a 98% concentrated sulfuric acid solution per gram of sample, stopper the glass stopper, and gently shake the flask to fully wet the sample at different bath temperatures. It oscillates at different times. The residue was transferred together with a concentrated sulfuric acid solution to a beaker containing an appropriate amount of a 75% by mass sulfuric acid solution, and then the residue was filtered through a glass wool core of known dry mass and evacuated by vacuum suction. The cold water was washed several times in a row, diluted twice in dilute ammonia water, and then washed several times with cold water, and drained by suction. Finally, the crucible and the residue are dried, cooled, weighed, and the percentage content of each of the polyester fiber/polyethylene fiber is calculated. 2 test results and data analysis The percentage of net dry content of the test results is calculated by the following formula: P1= P2=100-P1 Where: P1 - the percentage of net dry content of polyethylene fiber, %; P2—the percentage of net dry content of polyester fiber, %; M0—the dry weight of the mixture before dissolution, g; M1 - dry weight of the residue (polyethylene fiber), g; d—The mass correction coefficient of the polyethylene fiber after dissolution. The results of this test are calculated using 1.0. The difference between the test results and the artificial ratio is shown in Table 1. Table 1 Note: The above data are average values ​​of 10 parallel samples.  3 conclusions The test proves that the polyester fiber/polyethylene fiber mixed product is quantified by using 98% concentrated sulfuric acid. The results obtained under various common test temperature and dissolution time are close to the actual ratio, as shown in Table 1. ) shown. If 25 ° C and 10 minutes are selected as test conditions, it is more efficient and energy-saving. Moreover, the test results under this test condition are the closest to the actual ratio, and the absolute error is within 1%, which meets the requirements of relevant standards. Therefore, 25 ° C, 10 min can be used as the best test conditions. This method is highly operability and does not require special reagents or equipment, making it a viable method for such products. references: [1] GB/T2910.1–2009 Textile quantitative chemical analysis - Part 1: General rules [2] GB/T2910.11–2009 Quantitative chemical analysis of textiles - Part 11: Mixtures of cellulose fibres and polyester fibres [3] GB/T2910.24–2009 Textiles - Quantitative chemical analysis - Part 24: Mixtures of polyester fibres with certain other fibres ( phenol / tetrachloroethane method) [4] FZ/T01057.4–2007 Textile fibre identification test methods - Part 4 Â
Inoculating loop and needle are instruments used for inoculation, serial dilution, aseptic sampling, transfer and microbial sample coating. Options include color-coded disposable consumables made of materials such as polymers, aluminum, and other metals. The disposable inoculating loops and the inoculation needles are made of non-toxic (USP VI grade) polystyrene material, which can provide users with a smooth surface of the inoculation loop for use in vaccines and diagnostic kits, pharmaceuticals and biotechnology.
Sterile Loop,Inoculating Loop And Needle,Disposable Inoculating Loops,Sterile Inoculating Loop,Sterile Disposable Inoculating Loops Yong Yue Medical Technology(Kunshan) Co.,Ltd , https://www.yongyuecultureflask.com