The Chevrel phase compound is a molybdenum-based chalcogenide compound composed of Mo6T8 or MxMo6T8 (M is a transition metal and T is S, Se or Te). In the Chevrel phase structure, six Mo atoms are located at the six face centers of a cube, forming an octahedral Mo6 atomic cluster, and eight T atoms occupy the eight corners of the cube. Large three-dimensional open channel. Because of this unique structure, Chevrel phase compounds are used in superconductivity, thermoelectricity, catalysis, and batteries. Since the Chevrel phase Mo6S8 was first applied to the positive electrode of magnesium batteries in 2000, its application range has been broadened to almost all secondary battery systems. To this day, Chevrel Mo6S8 is still the most successful cathode material for magnesium batteries. But large-scale, high-quality synthesis of Chevrel phase Mo6S8 nanomaterials still faces great challenges. Current methods include solid-phase method, molten salt method, self-transporting high-temperature method, high-energy ball milling method, and two-step solution method synthesis, which have problems of large energy consumption, impure products, and inability to control particle growth. At present, the most commonly used solid-phase method uses CuS and MoS2 as sulfur sources to seal the reactants into a joint stainless steel tube filled with argon and react at 900 degrees Celsius for 24 hours. However, this method can only synthesize Mo6S8 with micron size, and because CuS will decompose at high temperature to generate sulfur vapor and escape, resulting in the formation of impurity MoS2. In view of this, Mao Minglei, Ph.D. student and Lin Zejing, group E01, Clean Energy Laboratory, Institute of Physics, Chinese Academy of Sciences / National Research Center for Condensed Matter Physics, Beijing, and under the guidance of Associate Researcher Suo Minmin, synthesized large-scale gas-phase mass transfer reactions using , High purity Mo6S8 nanosheets. Using Cu, Mo, and MoS2 as reactants, the decomposition of the reactants and the escape of sulfur vapor are avoided. Iodine is used to adjust the kinetics of the solid-phase reaction, which reduces the reaction temperature and time, and initiates preferential planar growth of Mo6S8 to form nanosheets. As a typical three-dimensional material, nanosheet Mo6S8 was obtained for the first time. In magnesium batteries and aluminum batteries, the Mo6S8 nanosheets have faster ion insertion kinetics and better electrochemical performance than microparticles synthesized by traditional methods. The results of this study were published recently on ACS Nano (ACS Nano, 2019, DOI: 10.1021 / acsnano.9b08848). The article is titled Iodine Vapor Transport-Triggered Preferential Growth of Chevrel Mo6S8 Nanosheets for Advanced Multivalent Batteries. The research team first explored the optimal synthesis conditions. By XRD testing the products at different reaction temperatures and reaction times, it was found that 800 degrees Celsius and 24 hours were the best synthesis conditions. At the same time, further research on the reaction path revealed that iodine vapor first reacted with copper to form CuI, and then reacted with Mo elemental and MoS2 to generate intermediate product Cu2Mo6S8. The intermediate product was further pickled to produce the target product Mo6S8 nanosheets. Then the author used XRD, SEM, TEM, STEM and other means to confirm the crystal phase and morphology of the synthesized Mo6S8 nanosheets. Subsequently, Mo6S8 nanosheets were applied to magnesium batteries and aluminum batteries. Mo6S8 nanosheets exhibited faster reaction kinetics, excellent cycle stability, and good low-temperature performance. In addition, the research team also used ex-situ XRD, EDS, and XPS to prove that Mo6S8 nanosheets undergo a significant phase change during the insertion and extraction of magnesium ions, and the charge transfer begins with sulfur ions and then transitions To molybdenum ions. In addition to being applicable to battery materials, Mo6S8 nanosheets can be widely used in superconductivity, thermoelectricity, and catalysis due to their high specific surface area, significant anisotropy, and unique surface properties. The gas-phase mass transfer reaction of iodine will provide a completely new route for large-scale synthesis of inorganic compounds. Related work has been supported by the National Key Research and Development Program (2018YFB0104400), the National Natural Science Foundation of China (51872322; 21905299), the China Postdoctoral Science Foundation (2019TQ0346), and Shell (PT76419).
PVDF is short for Polyvinylidene Fluoride, it is a kind of Fluoride plastic with excellent physical mechanical and chemical properties, PVDF chemical stability make it no reaction with other substances, in this case it have excellent corrossion resist property and can be used for strong acid. In this case PVDF Diaphragm Valve is most suitable for chemical industrial, semi conduct industria. The working temperature could up to 140 degree. Standard fit for ANSI, JIS, DIN, pressure rate PN10 bar. Manual operate and pneumatic actuator operation both avaliable.
Ningbo RMI Plastic Co.,Ltd offer PVDF diaphragm valve with specification:
Size range:DN15~DN250 (1/2 inch ~ 10 inch) PVDF Diaphragm Valve,PVDF Diaphragm Valves,Diaphragm Valve PVDF,PVDF Valve Ningbo RMI Plastic Co.,Ltd , https://www.rmiplast.com
Figure 1. Schematic diagram of the synthesis of Mo6S8 nanosheets by vapor-phase mass transfer reaction of iodine. 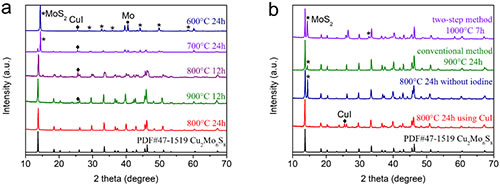
Figure 2. Using XRD to explore the optimal conditions and reaction path for the synthesis of Cu2Mo6S8. 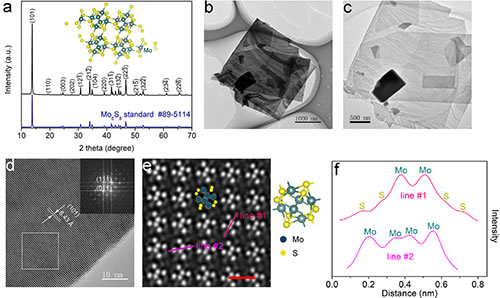
Figure 3. Characterization of Mo6S8 nanosheets 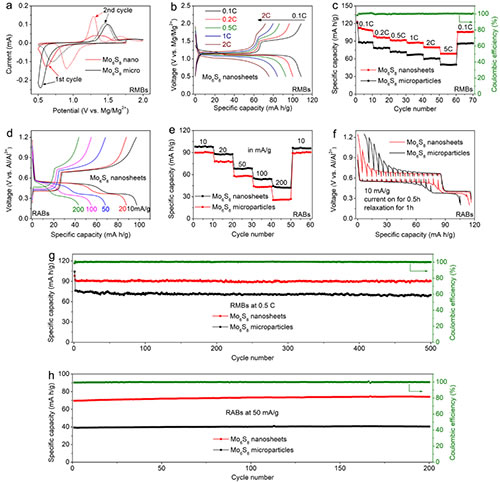
Figure 4. Electrochemical performance of Mo6S8 nanosheets in magnesium batteries and aluminum batteries
Body materials:PVDF
Diaphragm material:PTFE,EPDM,FPM,NBR,NR
Connection: Flange ANSI CL150, JIS10K, DIN PN10; Socket: DIN, ANSI, JIS
Design pressure:0.25~1.0Mpa (PN2~10 bar)